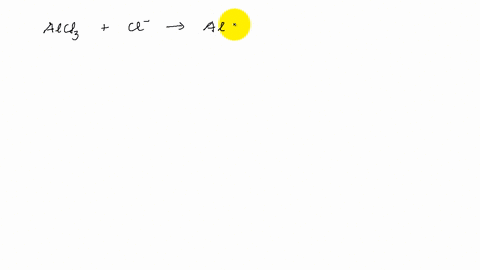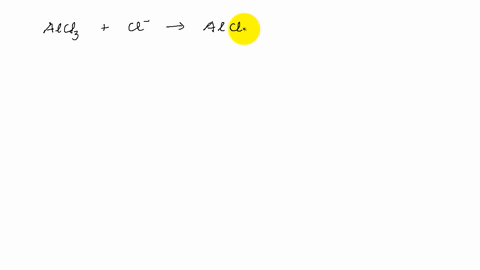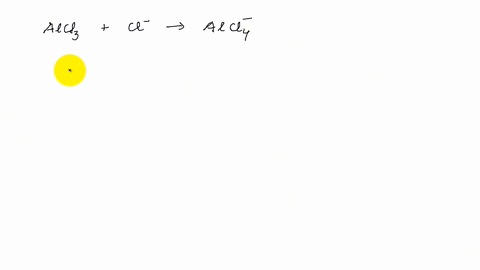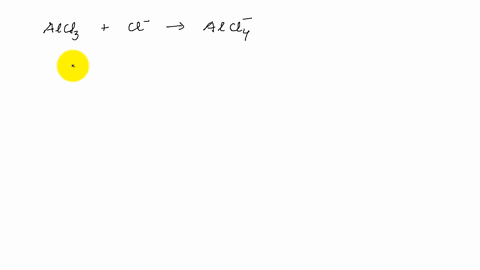When BCl3 is treated with water, it hydrolyses and forms [B[OH]4]^– only whereas AlCl3 in acidified aqueous solution forms [Al (H2O)6]^3+ ion. - Sarthaks eConnect | Largest Online Education Community

Describe the change in hybridization of the Al atom in the following reaction Alcl3+Cl- gives AlCl4- - YouTube

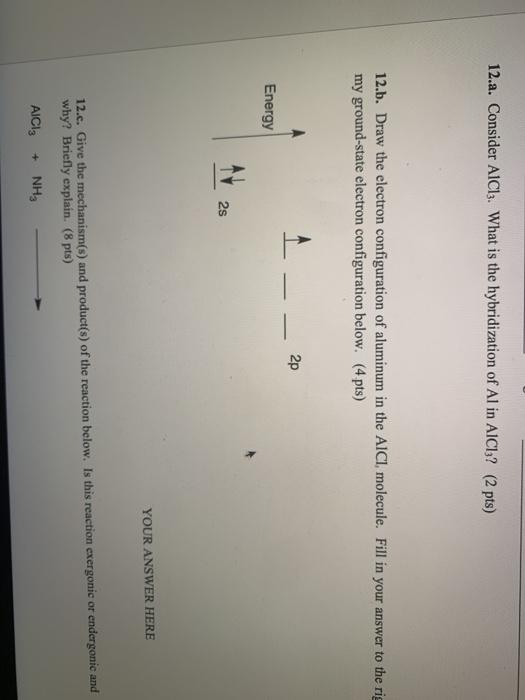
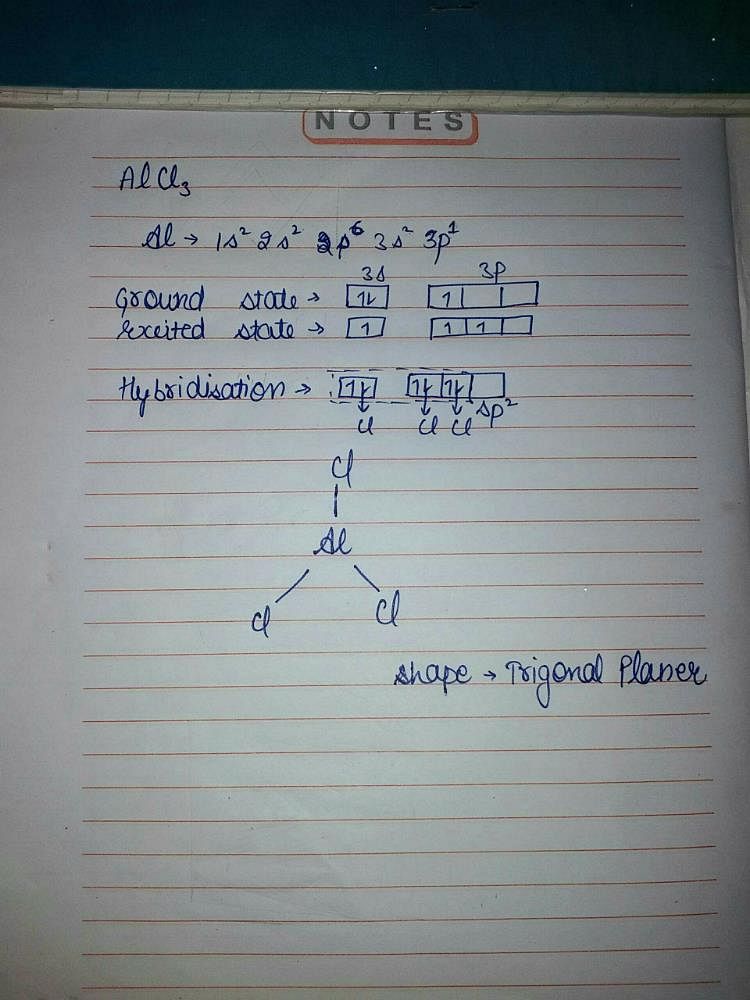
What is the molecular structure of the molecule AlCl3? What characteristic is notable about the structure that helps us to understand the acidic nature of AlCl3? Write a chemical reaction between AlCl3

inorganic chemistry - Explanation of bond angles in the aluminium chloride dimer - Chemistry Stack Exchange
6. Which one of the following compounds has the electron pair geometry as the trigonal bipyramid with three equatorial positions occupied by lone pairs of electrons? A) [AlCl3] B) XeF2 C) [

Mention the hybridization of the central atom in the given molecule. Molecular AlCl3 | Homework.Study.com
4. q hybridisation of Al in AlCl3 (monomeric form above 800C) and Al2Cl6(dimeric form below 400C) respectively are?
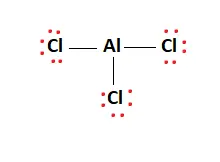
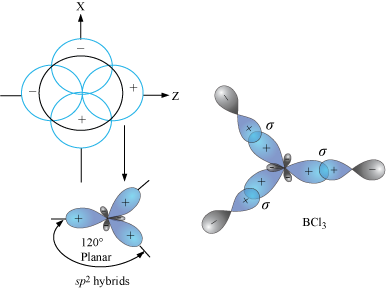
AlCl3 Lewis Structure, Molecular Structure, Hybridization, Bond Angle, and Shape - Geometry of Molecules

AlCl3 lewis structure, molecular geometry, polar or nonpolar, hybridization, bond angle | Molecular geometry, Molecular, Molecular shapes