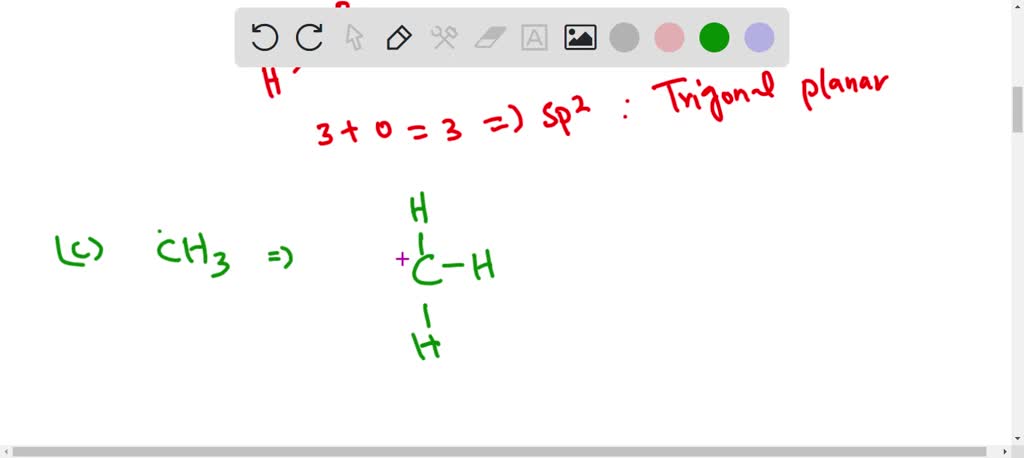
Discuss the orbital structures of the following molecules on the basis of hybridisation (i) BH3 (ii) C2H2 please answer fast - Chemistry - Chemical Bonding and Molecular Structure - 15719827 | Meritnation.com
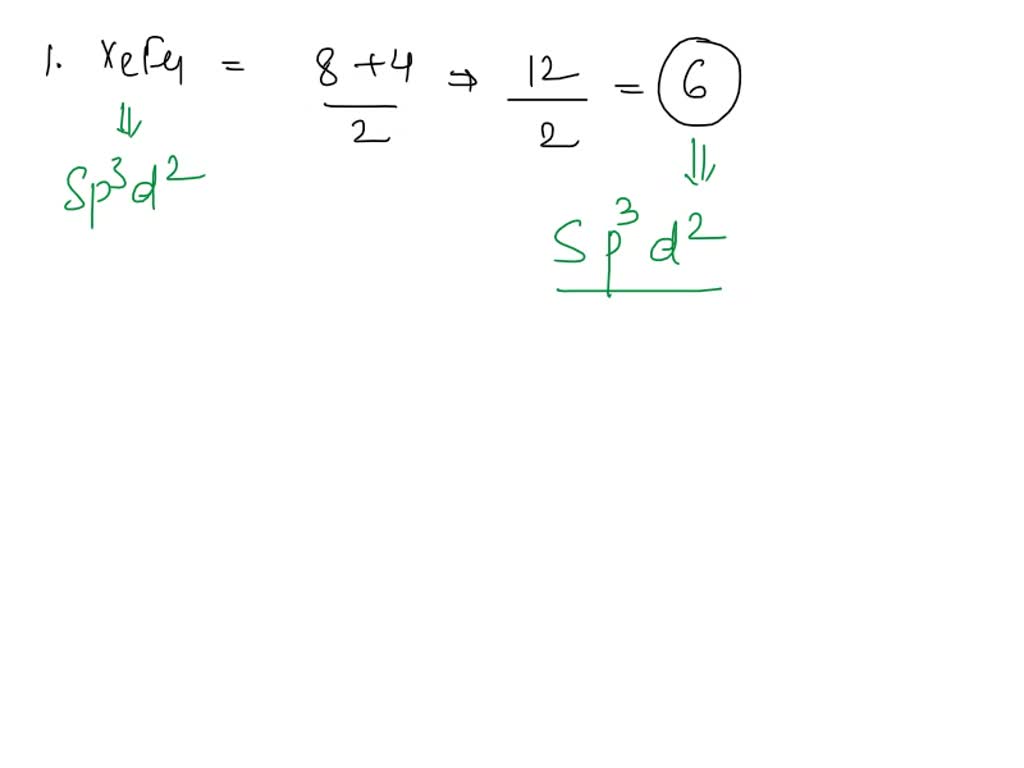
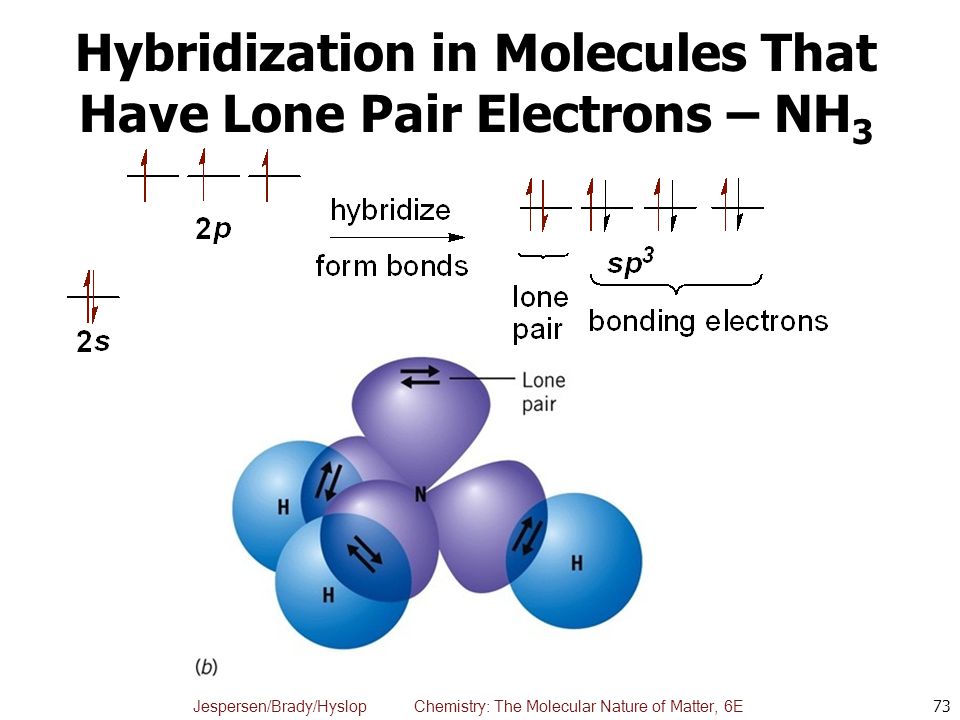
SOLVED: Give the expected hybridization of the central atom for the following molecules or ions: 1. XeF4 2. PH3 3. SO4^2- 4. BH3 5. PO3^3- 6. ClO2- 7. SO4^2-

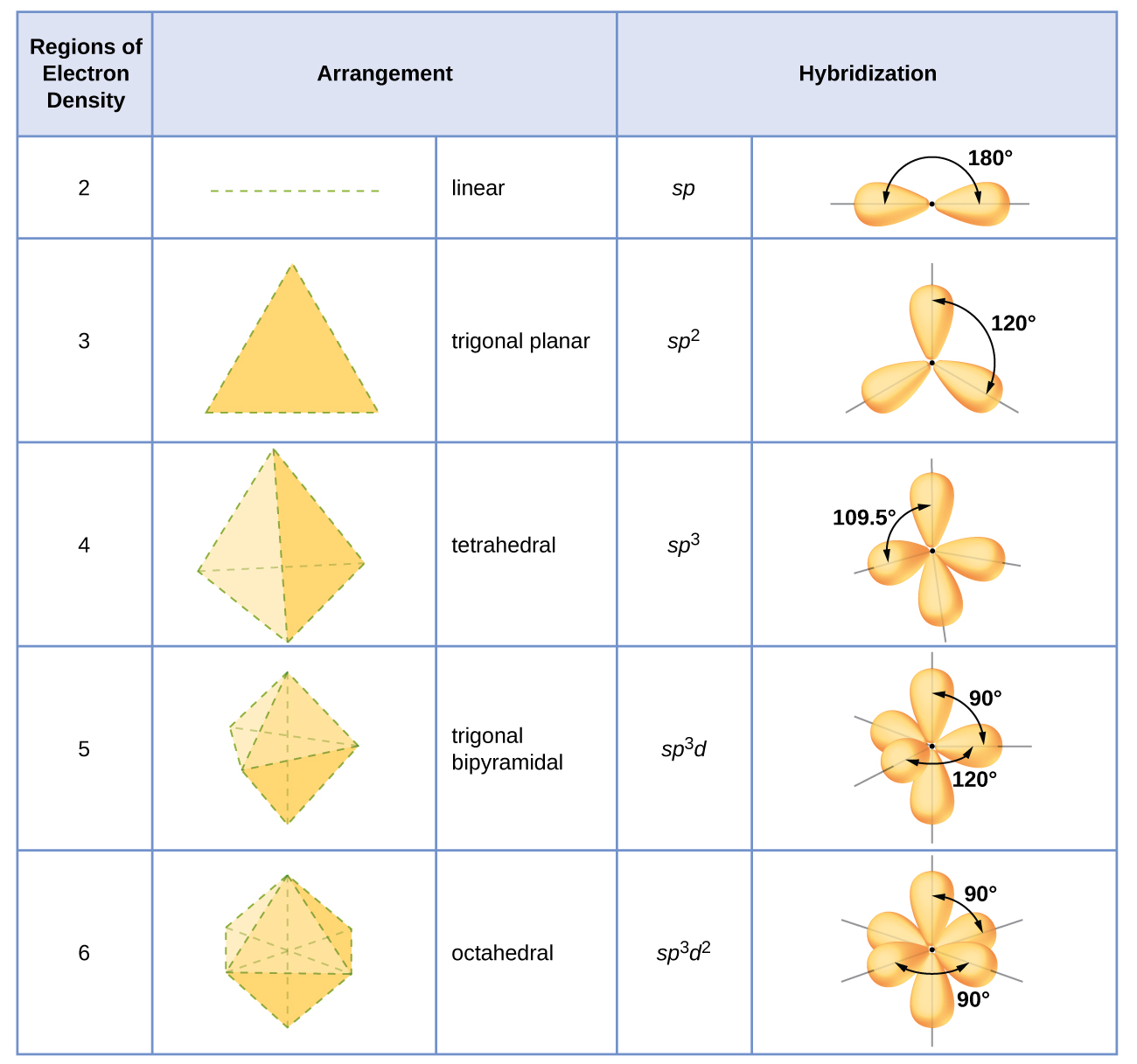
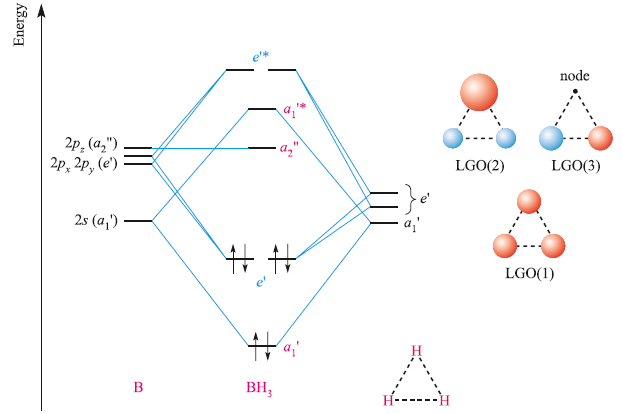
The state of hybridisation of central atom in dimer of B{H}_{3} and Be{H}_{2}?s{ p }^{ 3 },s{ p }^{ 3 }s{ p }^{ 3 },s{ p }^{ 2 }s{ p }^{ 2 },

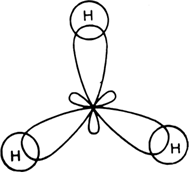
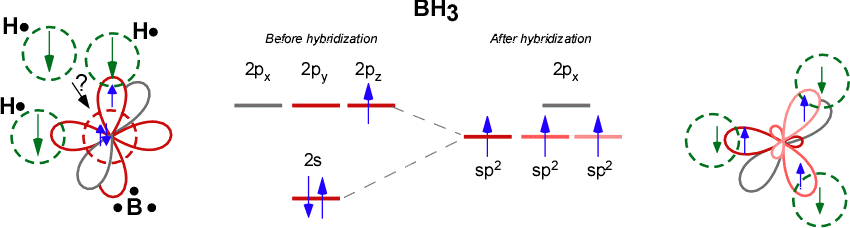
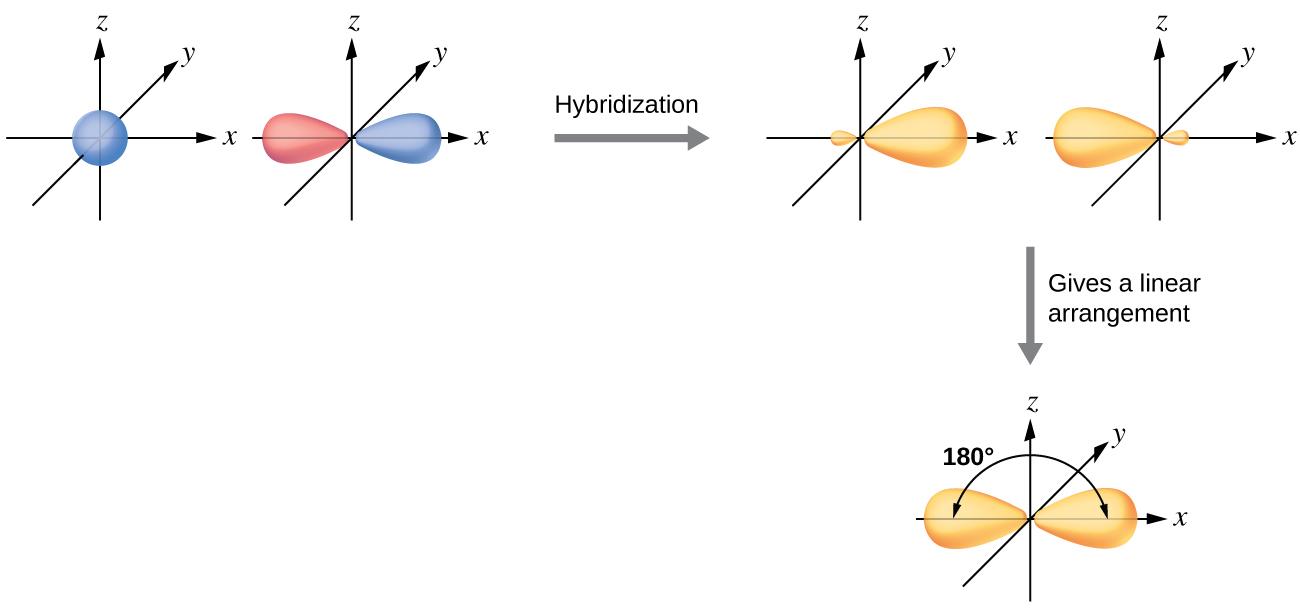
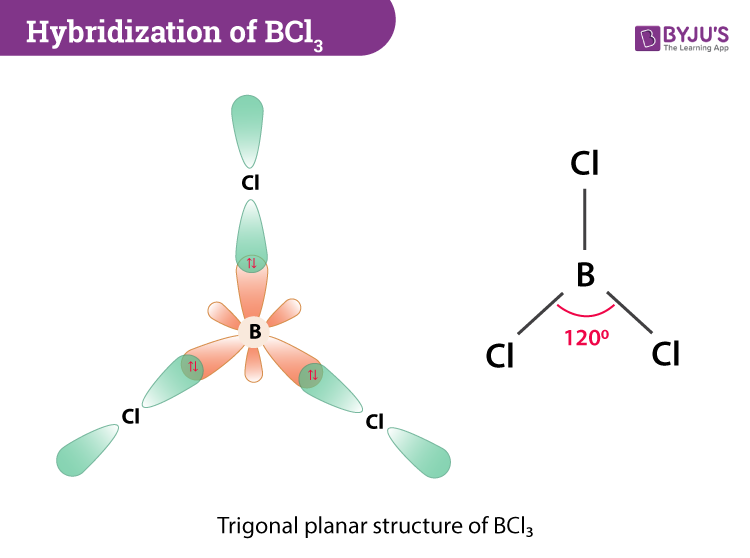
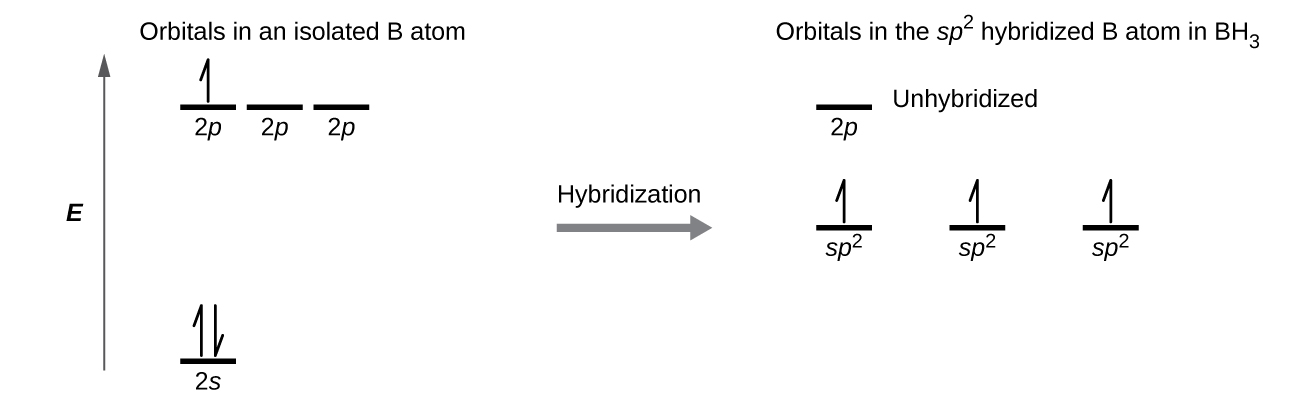
SOLVED: (a) Using the lens pond theory, specify the type of hybridization on the central atom in the BH3 molecule. sp2-hybridization hybridization sp-d-hybridization (b) Draw orbital diagrams showing the distribution of orbitals,
Discuss the orbital structures of the following molecules on the basis of hybridisation, (i) BH3 (ii) C2H2 - Science City - Quora

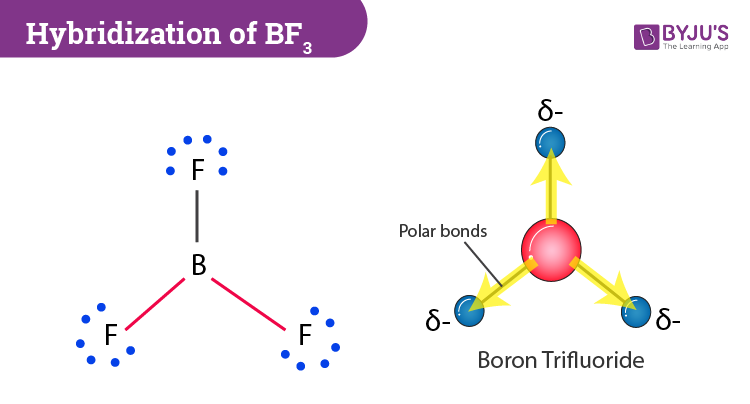
The B-H bond distances are about the same in BH3 and BH4-. however, the B-F bond distance in BF3 is shorter than that in the BF4- ion. Explain. | Homework.Study.com
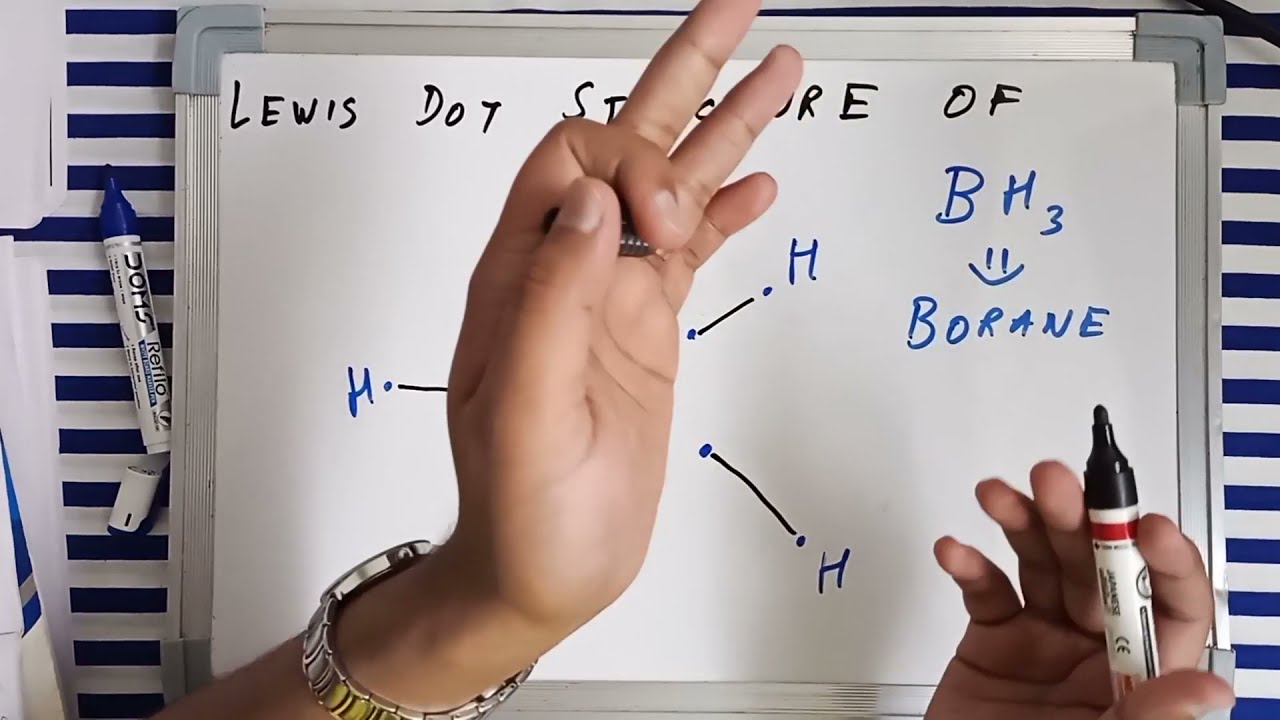
Lewis dot structure and hybridisation of BH3 | Borane lewis structure and hybridization | JEE | NEET - YouTube