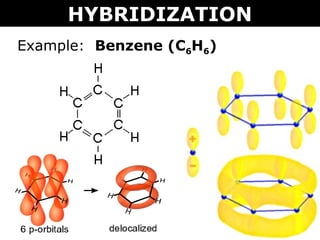
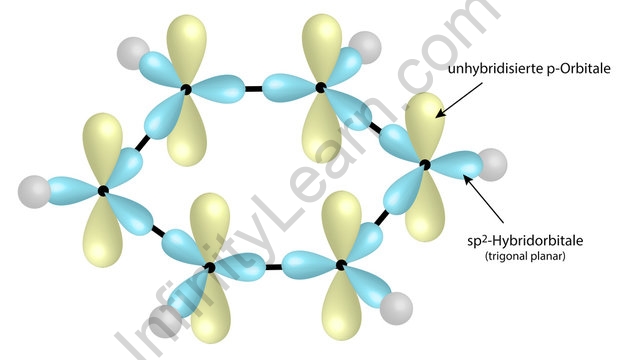
Why does benzene form sp2 hybridisation and not sp3? Why does one zth 2p orbital not participate in hybridization? Can someone explain this briefly? - Quora
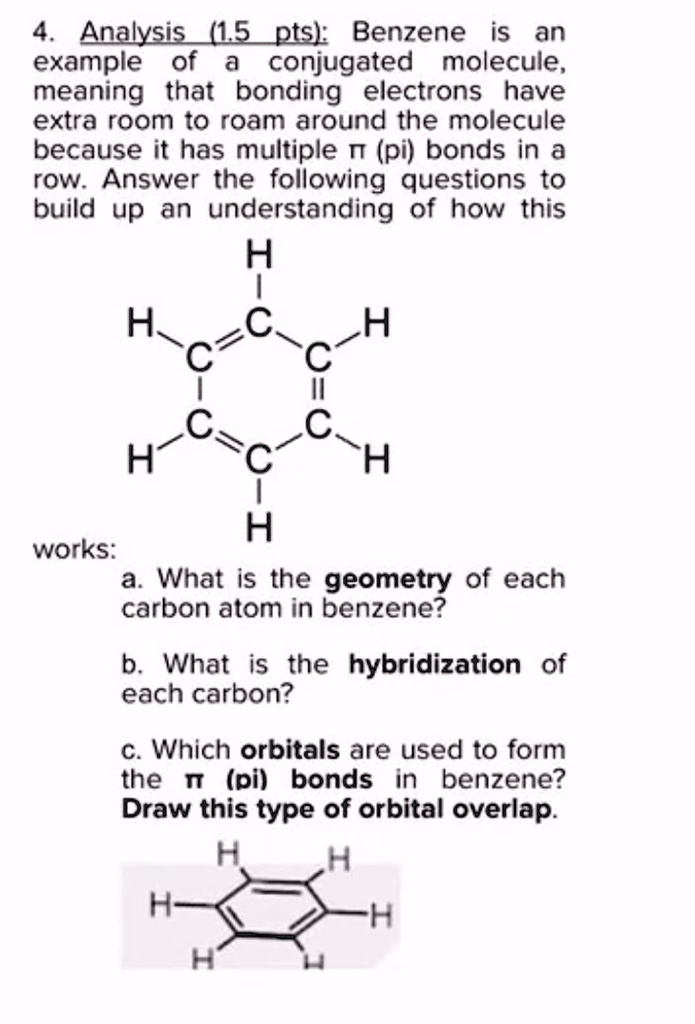
SOLVED: Benzene is an example of a conjugated molecule, meaning that bonding electrons have extra room to roam around the molecule because it has multiple π (pi) bonds. In order to understand
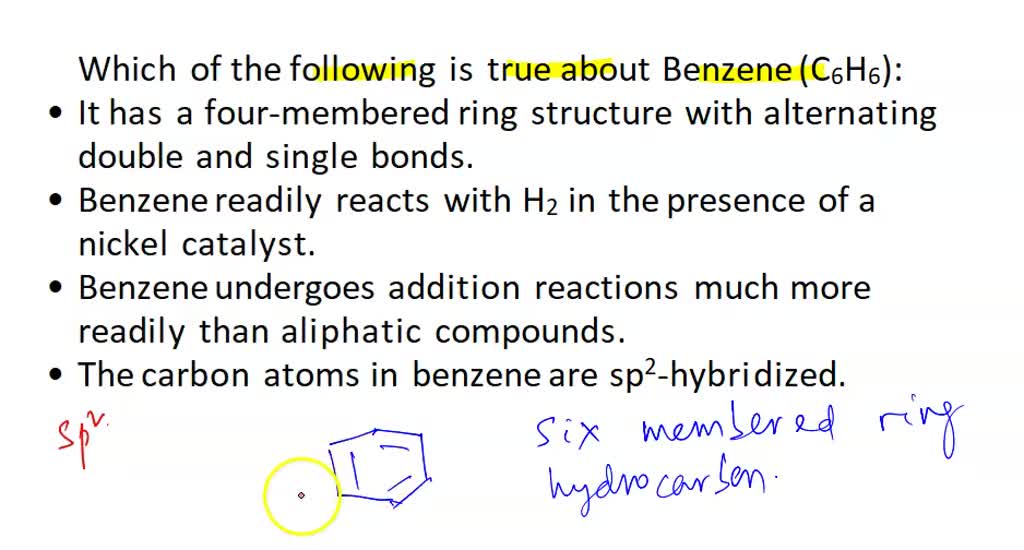
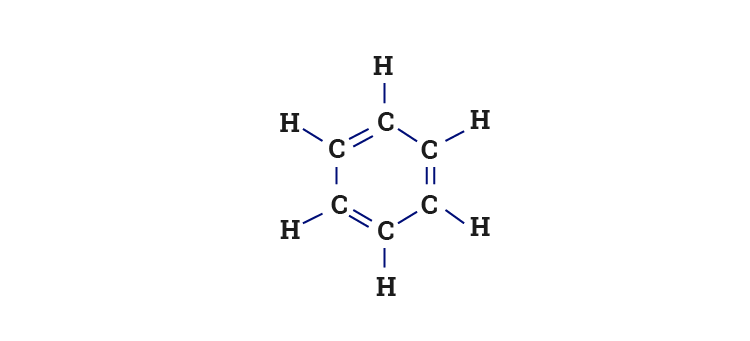
SOLVED: Which of the following is true about Benzene (C6H6)? It has a six-membered ring structure with alternating double and single bonds. Benzene readily reacts with H2 in the presence of a

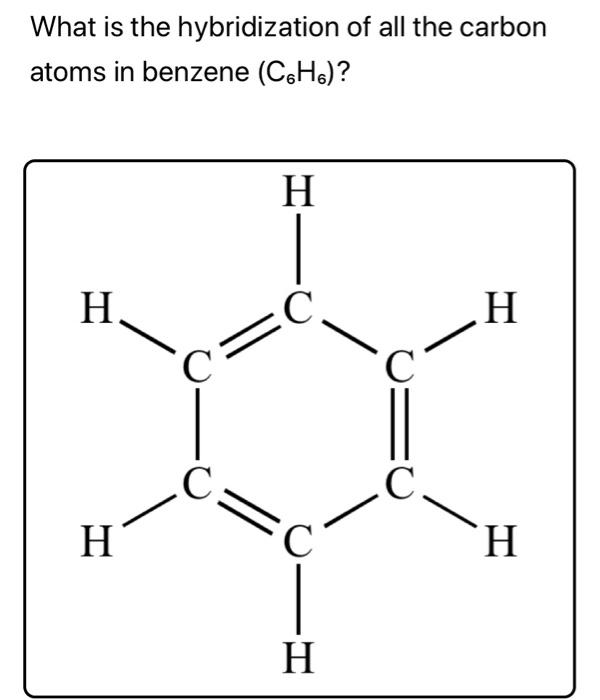
Benzene C₆H₆ : Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure – infographic

C6H6 lewis structure, molecular geometry, bond angle, hybridization | Molecular geometry, Molecular, Molecular shapes





![The number of \\[s{p^3} - s\\] sigma bonds in benzene is:A.3B.6C.12D.none The number of \\[s{p^3} - s\\] sigma bonds in benzene is:A.3B.6C.12D.none](https://www.vedantu.com/question-sets/6c01f233-1562-4d6e-b183-b09dcf51bc951429559277162239695.png)