![OneClass: Cu in [Cu(CN)4]2- and [Cu(NH3)4]2+ has d9 electron configuration ,so the geometry should be... OneClass: Cu in [Cu(CN)4]2- and [Cu(NH3)4]2+ has d9 electron configuration ,so the geometry should be...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/130/13010410.webp)
OneClass: Cu in [Cu(CN)4]2- and [Cu(NH3)4]2+ has d9 electron configuration ,so the geometry should be...
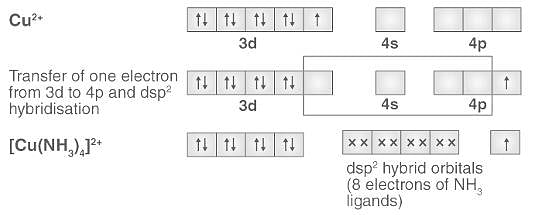
Cuprammonium ion has ______ shape.a)Octahedralb)Tetrahedralc)Trigonald)Square planarCorrect answer is option 'D'. Can you explain this answer? - EduRev Chemistry Question
![PDF) Notizen: E.P.R. determination of the hybridisation and the H-N-H bond angle of ammonia in [Cu(NH3)4][PtCl4] PDF) Notizen: E.P.R. determination of the hybridisation and the H-N-H bond angle of ammonia in [Cu(NH3)4][PtCl4]](https://i1.rgstatic.net/publication/276530089_Notizen_EPR_determination_of_the_hybridisation_and_the_H-N-H_bond_angle_of_ammonia_in_CuNH34PtCl4/links/595bee96458515117741bdc3/largepreview.png)
PDF) Notizen: E.P.R. determination of the hybridisation and the H-N-H bond angle of ammonia in [Cu(NH3)4][PtCl4]

Explain the Geometry of Cu(Nh3)4 2+ on the Basis of Hybridisation At. No. Cu = 29 - Chemistry | Shaalaa.com
Explain geometry of [Cu(NH3)4]^+2 on basis of hybridization. - Sarthaks eConnect | Largest Online Education Community


![Solved] what is ths hybridization of cu in cu[(nh3)4]2+ - Brainly.in Solved] what is ths hybridization of cu in cu[(nh3)4]2+ - Brainly.in](https://hi-static.z-dn.net/files/de4/49aeb4fd2144e40e1aba99873c3f2a73.png)

![why [Cu(NH3)4]2+ form Square planer complex? [Cu(NH3)4]2+ वर्ग समतलीय क्यों बनाता है? - YouTube why [Cu(NH3)4]2+ form Square planer complex? [Cu(NH3)4]2+ वर्ग समतलीय क्यों बनाता है? - YouTube](https://i.ytimg.com/vi/KHpuu0UJwbw/maxresdefault.jpg)
![Hybridization and magnetic moment of `[Cu(NH_(3))_(4)]^(2+)` and `[Mn(CN)_(6)^(3-)` ions - YouTube Hybridization and magnetic moment of `[Cu(NH_(3))_(4)]^(2+)` and `[Mn(CN)_(6)^(3-)` ions - YouTube](https://i.ytimg.com/vi/owlgbfjVOzc/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLBv6TqxUZtnijDOJn7QLMuUGig2-A)

![What is hybridization of [Cu (NH3)4 ]2+,[PtCl4]2-||Exception in coordination compound|| - YouTube What is hybridization of [Cu (NH3)4 ]2+,[PtCl4]2-||Exception in coordination compound|| - YouTube](https://i.ytimg.com/vi/4uNg5AAJJkA/sddefault.jpg)
![Explain the geometry of [Cu(NH(3))(4)]^(2+) on the basis of hybridisa Explain the geometry of [Cu(NH(3))(4)]^(2+) on the basis of hybridisa](https://d10lpgp6xz60nq.cloudfront.net/physics_images/GRK_10Y_SP_HSC_CHE_JLY_18_E02_001_S01.png)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-2.png)

![Explain the geometry of [Cr(NH3)6]3+ ion by valence bond the Explain the geometry of [Cr(NH3)6]3+ ion by valence bond the](https://www.zigya.com/application/uploads/images/chen12070390_5714b9d19ba87.png)